LIGHTSTICKS
CYALUME® lightsticks, manufactured by American Cyanamid Co., and
similar items available from other manufacturers, are devices that
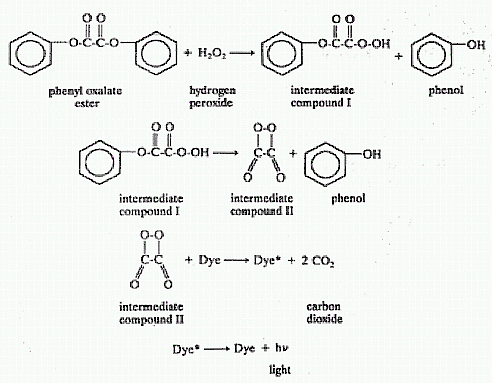
produce a “cool-light” by means of a chemical reaction. The reaction is
similar to the one that produces light in a firefly, but the chemicals
involved are different. A lightstick consists of dilute
hydrogen
peroxide solution in a phthalic ester solvent contained in a thin glass
ampoule which is surrounded by a solution containing a phenyl oxalate
ester and the fluorescent dye 9,10-bis(phenylethynyl)anthracene, (blue
lightsticks use 9,10-diphenylanthracene) all contained in a plastic
tubular container.
When the glass ampoule
is broken, by
bending the
lightstick, the hydrogen peroxide and the phenyl oxalate ester react to
form phenol and some intermediate (short lived) compounds. During the
reaction, the energy given off
is transferred to the dye molecules.
The excited dye molecules (designated as Dye®) give off the excess
energy in the form of light without any noticeable heat. Thus
the
name, “cool-light”.
Lightsticks can be used to demonstrate how the rate of a chemical
reaction varies with temperature. To do this, initiate three
lightsticks. Place one lightstick in ice water, one in hot tap water
(not boiling), and leave one at room temperature as a control. The
effect of temperature on the reaction can be observed within a few
minutes.
Lightsticks are used as emergency lights and safety lights in industry
and for camping. As toys, they are made in the form of earrings,
necklaces, bracelets, rings, eyeglasses, and bowties. They are also
used to light up balls, golf balls, and flying disks for nighttime
playing.
A recent variation of lightsticks are Magic in the Night® light
shapes.
These are adhesive backed packets in various shapes, such as circles,
diamonds, and stars, and filled with lightstick chemicals which are
stored in separate glass ampoules. Both ampoules must be broken in
order to mix the chemicals.
Lightsticks are dated to indicate their life time. If stored in a cool
place, they remain active for at least one year past that date. Light
shapes have no expiration date.
The description above was taken from Chemistry
in the
Toy Store and used with the permission of the author David A.
Katz.
Graphic is from Shakhashiri, Chemical Demonstrations, Vol. 1,
Univ. Wisconsin Press, 1983
Go
to our Science
Fun page
Go to our Travels page
Go
to our Personal
home page
Go
to our Community
page
E-mail
Nancy
and Alan
