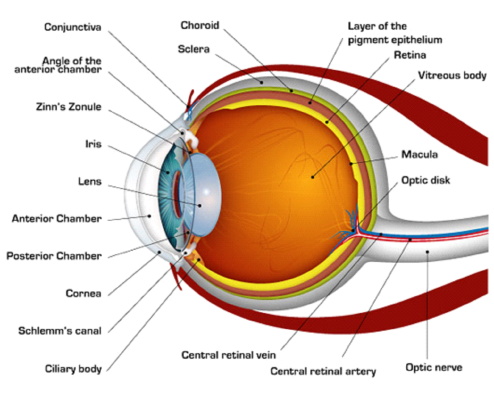
Here is a diagram of the eye with the names of its parts.
Optical illusions and hidden pictures show how the eye can be fooled.
The kids here are looking for a baby in this picture.
Some found it immediately others had to have it pointed out to them.
Here is a copy for you to try your powers of observation.

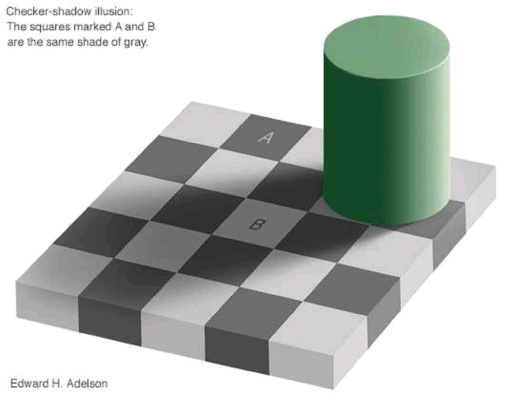
Perception of light and dark areas depends strongly on what is around them..
Two gray areas (A and B) are the same.
If you don't believe me make 2 holes in a sheet of paper so that you can only see those 2 areas.
When you cover the rest you will see that they are the same.
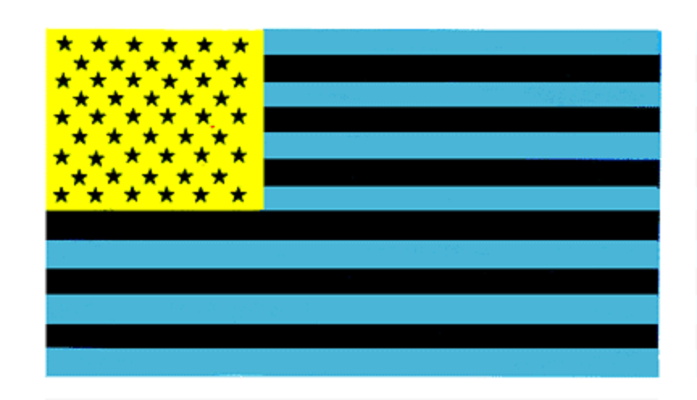
Sometimes your eyes will tire of seeing some colors and will surprise you when you look at a blank white area.
Here this strangely colored flag can be used to demonstrate the effect.
To see the effect stare at the lower right corner of the yellow part of the flag for 30 seconds or more.
Try to keep your eyes focused on exactly the same spot.
Now look at a blank sheet of paper or wall to see what colors you see.
I think this fellow was surprised.
The older kids investigated spectra.
I aimed three laser pointers at the same spot on the left of this picture then used a diffraction grating to separate their colors.
The first cluster of three spots show that blue (which has the shortest wavelength) isn't deflected as far as the green and red with the longest wavelength is deflected the most.
The same pattern is repeated with a wider spread in the second order spots on the right of the picture..
Each photon of the shorter wavelengths (blue and ultraviolet) carry more energy than those with longer wavelengths (other visible colors and infrared)
This video shows the effect of three different colors of laser light on beads that react when sufficiently energetic photons hit them.
Even though the green laser is much brighter photons from it don't deposit enough energy in the beads to have any effect on them.
We can see the colors that are produced by elements when they are excited by fire.
Here we have the chlorides of strontium, potassium, copper, calcium, and sodium,

Here you can see the spectra of the copper (cupric chloride) flame.
The central flame is a bright green so it is overexposed and looks nearly white except around the edges.
The diffraction grating separates the colors in the eight images around it.
They are most easily seen at sharp edges of the flame.
Spectra can also be produced when high voltage electric current is passed through gaseous elements in discharge tubes..
Here are what the unexcited tubes look like.
And here is the spectra produced by two of the tubes
The nearly white line on the left of this picture is what you see before the lines have been separated and those to the right are the spectra of helium.
Xenon discharge tube
The kids used defraction gratings to make spectroscopes.
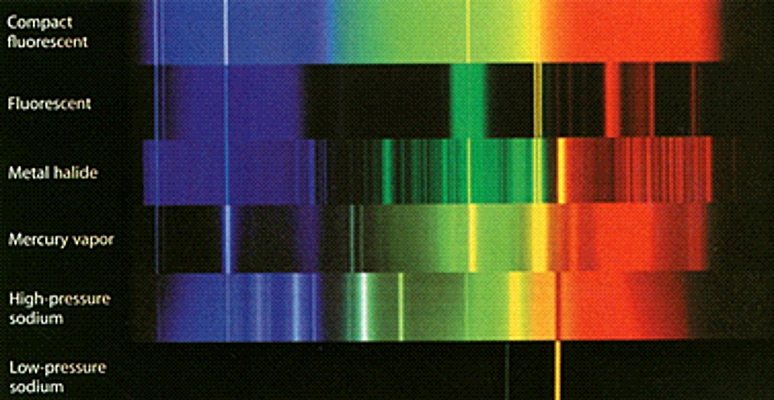
Here are the spectra of several different lights.
By comparing what they see looking through their spectroscope they can figure out what kind of light they are looking at.
Spectra can be used to identify elements in distant light sources.
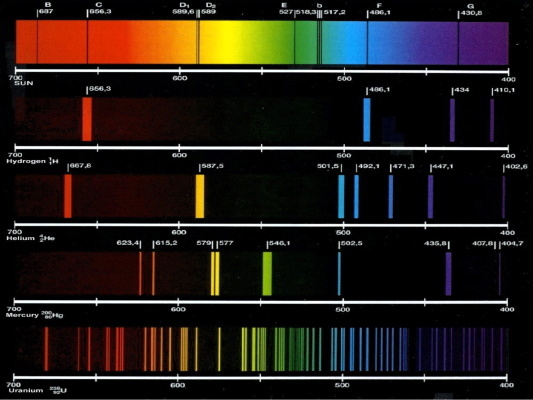
Individual elements can be identified in mixtures.
Here the dark lines show what you see if an incandescent light such as the sun or the filament of an old style light bulb passes through a gas.
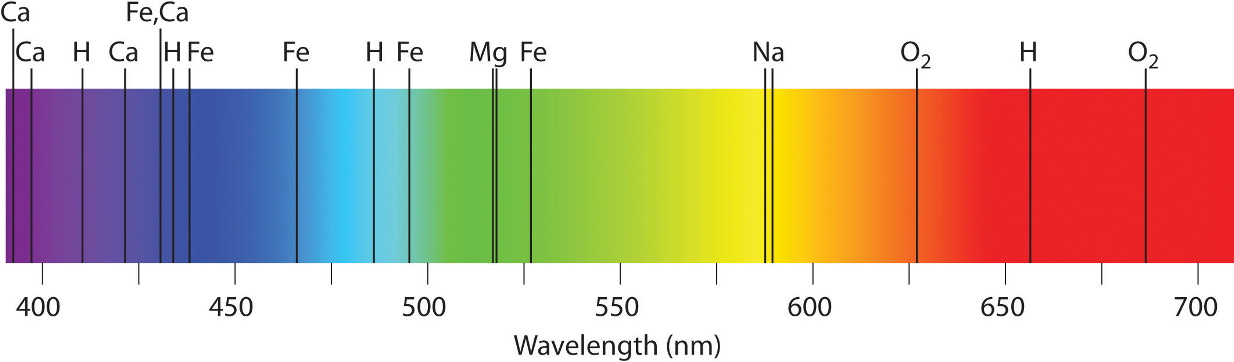
Here is a solar spectrum.
In order to show all the lines it has been broken into parts.
Each horizontal band should be joined to the right end of the one above it to make a single very long spectrum.
This is what it looks like in the room when the neon tube is excited.
Some of the kids are using the spectroscopes that they made to check its spectrum.
Here are pictures of the classes checking the spectra of various other light sources in their classrooms.





Science can be amazing and I love it when the kids react like this to one of our demonstrations.
