Here campers are identifying some of the things that can be found on a topographic map, also called a quadrangle.
They identified cliffs, rivers, strip mines, cemeteries, structures, roads, swamps, wells, hill tops, flat bottom lands, and many more features.
We have found the ability to read one of these maps can come in handy when planning a search for someone who has been reported lost.
With what we had learned about how topography is represented we were able to build a relief map of Sandy Hook.
We glued copies of the map to foam board and then they cut along one of the contour lines.
One of the kids cut along the 750 ft line on a map, another cut the 800 ft line on their map, with other of the kids doing the same for the other elevations.
We stacked all of the maps together to produce a relief map showing the hills around the town.
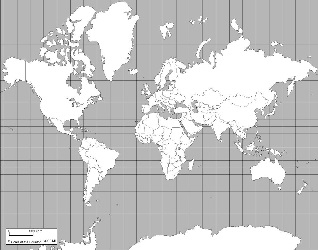
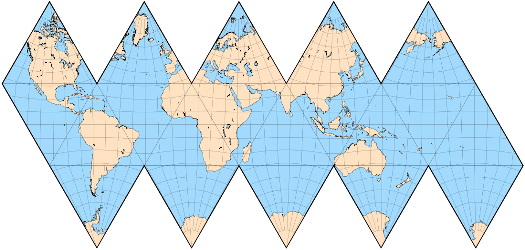
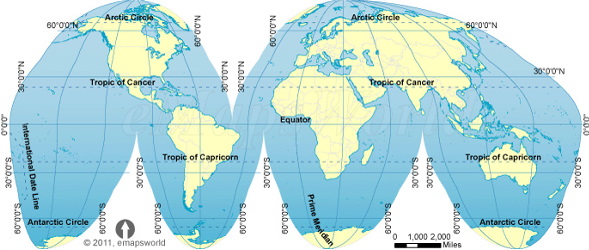
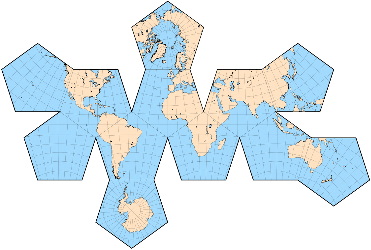
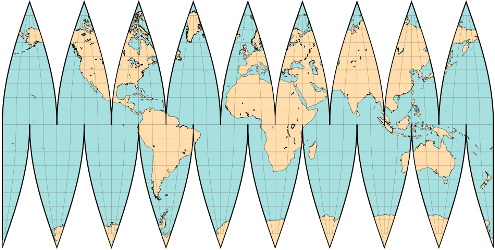
Here are some of the world maps we considered.
Note how different ones distort the areas because none of them can represent the surface of a sphere on a flat surface.
We then went outside to learn how to use a transit and plan tables to locate points that you can't get to when you are making a map.
They found that they could figure out the distance and direction of things in the school yard and well beyond it just by carefully measuring angles to each of them from two known points.
If you include a benchmark among the points you measure you can figure out the elevation above sea level of all the points on your map.
They also used road maps of Kentucky to plan a trip from Sandy Hook to Mammoth cave.
Some planned stops at many other points of interest in the state.
Another of the projects was the measurement of the acidity or alkalinity (acid or base) of various solutions.
The red and yellow rectangles on the table are pieces of goldenrod paper.
It is normally yellow but turns red when exposed to basic solutions then it will change back when an acid solution is applied.
They made secret messages of pictures with it too.
We also gave them filter paper that had been soaked in juice that had been extracted from red cabbage and then dried.
It would change from purple to blue when it was exposed to bases.
We also demonstrated some liquids that can be used as indicators including juice from grapes and red cabbage.
And to be complete in our study we used ones that are more likely to be found in a chemical lab (phenylalanine and universal indicator).
We mixed 120 ml of a 4% solution of polyvinyl alcohol and a 30 ml of a 4% sodium borate solution.
You can imagine polyvinyl alcohol (PVA) molecules as resembling limp strands of spaghetti that are as long as a football field.
When dissolved in water they easily slide over each other.
The sodium borate molecules are much smaller, imagine pinhead size.
Each solution is nearly as runny as water.
However, when they are mixed the borate ion forms links with random spots on the PVA molecules and the mix becomes very gooey.
The mix is still almost all water (96%) so it is surprising that it's consistency has changed so much.
In fact it now will stretch if pulled slowly, break if stretched abruptly, flow out into a puddle if left on a flat surface, bounce if shaped into a ball and dropped a short distance, and shatter if thrown down hard.
Here are a few pictures of the process.
And some shots of the resulting slime, stretched and broken.
Here is a video of the goo.
Calcium carbide reacts with water and releases acetylene gas which can be ignited as it bubbles off.
The reaction also produces a paste of calcium hydroxide (a medium strength base) in the water that remains.
Here is a picture of a miner's lamp that uses the reaction to fuel its flame.

Here is the reaction in a small dish.
The acetylene bubbles burst into flame when provided with a source of ignition.
We cut a bit of sodium metal with a knife and then flattened it with the knife point to demonstrate that it was very malleable.
When we dropped it in some water another of its properties was revealed, it is very reactive.
It has a larger affinity for oxygen than hydrogen does so the sodium grabs the oxygen from water molecules releasing the hydrogen and enough heat to melt itself and ignite the hydrogen.
You can tell the metal has melted because it changes into a ball form the original flattened lump.
You can see the result below.
The kids were interested to see that simply dropping a bit of metal in water was enough to make a series of little explosions.
The gas wasn't confined so it wasn't a problem.
When I was in high school one of my classmates took a large lump of sodium and coated it with Vaseline and flushed it down the toilet.
It was soon reacting and producing hydrogen which exploded causing the water in all the toilets to erupt.
Fortunately no one was hurt but as you might imagine it was a mess.
They also liked to see the thermite reaction.
Here is the setup.
The tub is filled with sand and there is a frying pan holding a computer hard drive about a foot up.
The flowerpot at the top has a mix of powdered iron oxide (rust) and powdered aluminum.
When that is heated a reaction starts.
The flame from a lighter or torch isn't hot enough, you have to use something even hotter, a strip of magnesium or a sparkler.
Once the reaction starts the oxygen combines with the aluminum leaving metallic iron at about 4500 degrees Fahrenheit (2500 Celsius).
The kids took a look then moved back before I set it off.
Unfortunately we didn't get pictures of the molten iron dripping onto the hard drive in the aluminum pan but check the Wikipedia entry on thermite to see why the military may use it on computer equipment.
Here the reaction is complete and the kids have moved closer to see the result.
The disk drive was still burning.
When things cooled off here is what we had.
The molten iron melted both the disk drive and the aluminum frying pan.
That will keep the data on the drive from being read as well as keeping anyone from frying an egg in the pan.
On Wednesday we took a field trip to COSI in Columbus Ohio, a great science museum.
And one of the demonstrations there was Thermite.
This time I got a video.
Other attractions at COSI are the unicycle ride across an 84-foot cable while 17 feet above the the floor below.
We got there early and there wasn't a big line so I got to ride it this year.
It was fun and the kids enjoyed seeing me on it.
Rat basketball was another hit.
These black hooded rodents have been trained to shoot hoops, almost always dunking the ball.
The second picture is one making a shot.

The Fireworks show was loaded with physics, chemistry and explosions.
We were the only group that stayed for the electrostatics show.
So everyone that wanted to got to take part.
The experience was shocking.
On the way home some of the kids were doing string figures.
Trying to solve the Eight Queens Problem the next day.
They were trying to put 8 markers on a chessboard without any of them being on the same row, column or diagonal.
Learning to solder two wires together.
They did it very carefully.
Then they assembled printed circuit flashlights.
They used clip leads, batteries, knife switches, and motors to wire up circuits that did simple logic ("AND" and "OR" decisions).
They also learned how to count using binary numbers, the system that computers use.
On Friday we dissected rats.
Some of the kids were a little apprehensive and took a while to get started.
But before long everyone was taking part.
They had diagrams and checklists that listed all the bones, muscles, and organs they were to find.
One surprise was that one of the rats was pregnant with six embryos
These girls even disected them.
One of the parents brought a snake that they had killed and asked if we wanted it.
It was a perfect opportunity to see its internal organs and compare them to the rats.
Here you can see the inside of its body and how its muscles and ribs move.
Another round of puzzles and games.
This time the game is Hex.
Here they are using static electricity to light small fluorescent bulbs.
In the first one they have induced a charge on the aluminum pie pan and the lamp blinks when it touches the pan.
In the rest they are using a Van de Graaff.
It was generating about 300,000 volts that day.
You can see the spark from it in several of the pictures.
Not everyone was there when we took the group pictures but here you can see most of the crew.
