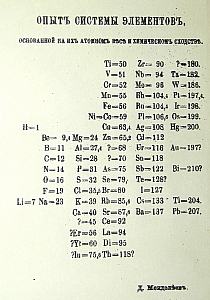Mendeleev
While
in St. Petersburg we decided to try to find Demitri Mendeleev's
laboratory. He was the scientist who had discovered the
periodic
table which brought order to the science of chemistry. While
we
were on a driving tour of the city our guide pointed out Mendeleev's
statue outside the the university where his lab was located.
We
had no problem finding the building again when we were on our
own.
Now our problems began. There were no signs indicating where
the
lab was in the building. Actually it was a complex of
university
buildings with no signs on any of them. We asked someone who
was
passing by. We of course couldn't ask in Russian and they
didn't
speak English. They did understand us well enough to lead us
to a
door in a dark hallway which we had walked past in the first building
we entered. They pointed to the unmarked door and
left. We
tried the door. It was locked. Not knowing quite
what to do
we were standing there when Nancy noticed what appeared to be an ornate
doorbell button that had been painted over many times. We
tried
it and heard a bell ringing inside. The door soon
opened
and we were met by a woman who of course spoke to us in
Russian.
We indicated we didn't understand. She said
"Francais?" We said "Niet, English." She
said
"Deutsch?" Again niet. She tried a couple more
languages
then shrugged, said something we took to be "Sorry." and began to close
the door. We protested and pointed out the shirt and socks I
was
wearing (both had a copy of the periodic table) and asked if we could
just look around. She gave a little laugh and ushered us
inside.
We found ourselves in a rather small museum which had been his lab and
office. As we started to look around she pointed out a
picture on
the wall and named the people in it. They were scientists
that I
had heard of so I nodded and apparently looked like I
understood. So began our tour narrated entirely in
Russian. There are a remarkable number of technical words
that
when said in Russian sound like English spoken with a strong Russian
accent. That, plus being able to recognize equipment and
materials Mendeleev used, a lot of gestures, and her patience with us
resulted in a very educational and enjoyable tour. I
shouldn't
leave you with the impression that we understood everything
though. At one exhibit our guide, apparently
encouraged by
our interest, launched into an extended discourse. I was
straining to pick up something which I recognized. She could
see
that I wasn't getting it and tried again, and again, and
again. Finally Nancy 's eyes brightened as she said
"Ahhh!" As we were lead to the next exhibit I asked Nancy
what
our guide had said. Nancy replied very quietly that she had
no
idea but it was clear that our guide was going to keep explaining till
we understood and that was the only way we would see the rest of his
lab.
This statue wasn't far from his lab but we would have been looking for
a long
time if
that was our only clue to its location. The wall
next to
the statue displayed the periodic table of the elements. The
symbols for the ones he predicted would be discovered are shown in blue.
Here is our guide
pointing out
Mendeleev with some of the famous scientists of the day. The
second picture shows him with some of his students. In the
last
he is at his lab desk pondering the puzzle of how to make a logical
arrangement of the 63 elements that were then known.
Here are several of the
periodic
tables that were on display. If you look closely at the first
handwritten one you will see chemical symbols and atomic
weights.
Note the question marks next to some entries. If there is no
chemical symbol it indicates his predicted weight for an element yet to
be discovered. Much has been made of those
predictions but
what is as impressive to me is the symbols and weight pairs he
questioned. Those question marks were saying that he thought
his
theory was better than the work of scientists who had made those
determinations. That strikes me a rather nervy. By
the way
I checked some of them and Mendeleev was right.
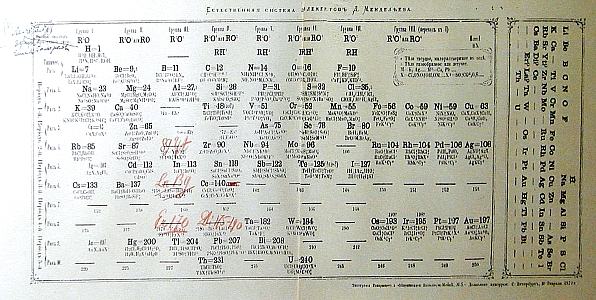
Here is a printed
version of his
table with some of the blanks filled in as the elements were
found. The second table shows some of his notes that helped
convince him that there were elements yet to be discovered.
I wasn't able to figure
out just what
this diagram was showing. There are 7 sectors with numbered
circles in them and lines connecting some of them. The
sectors
could correspond to the columns of the periodic table (minus the noble
gases which were unknown at that time) but the numbers don't seem to
match up with atomic weights. There are notations in Russian
that
would help to understand it I am sure but since I read Russian at an
early first grade level I will need some help. If you know
Russian or chemistry and would like to try just click the picture for a
high resolution version and then let me know what you figure
out.
The label at the bottom says something about a (logical topic/theory?
scheme) and I can't translate the rest.
Some of his chemicals on
display. I didn't try to read many of the labels but that
purple
one in the center says it is K Cl O4 which I think
should be a white
crystal. Maybe I missed a letter or two in the formula.
When I saw these I couldn't help thinking about the chemicals that were
in Edison's lab in New Jersey. Not long after I visited it,
many
years ago, they discovered there were many bottles of toxic and/or
potentially explosive chemicals there. I wonder if these have
been checked.
If you were doing
chemistry research
in the 1860's you may have been working with minerals to see if you
could isolate new chemicals and if you were very lucky or very good new
elements. How would you know if you had been
successful?
Only by checking the properties after you had purified it and comparing
those with all of those then known. The shape of the crystals
that are formed is one of those properties. Here we see 2
mineral
samples and 3 common crystal forms that were on display.


Equal volumes of gases
contain the
same number of atoms (if they are the same pressure and
temperature). This provides a way to compare the atomic
weight of
some elements. All we have to do is accurately measure the
volume, weight, temperature, and pressure of the element in
gaseous form. If they aren't gases maybe a compound of the
unknown with other already known elements is. Measure that
compound and do some arithmetic and you have the atomic weight of the
unknown. What if you can't find any compounds that are
gases. You could try to measure the amount of some material
that
you have already characterized it takes to completely react with your
unknown sample. If you do that accurately, and can figure out
the
chemical formulas for the reactants and the product, and measure the
weights of each, and do more arithmetic you have the atomic weight of
your unknown.
Simple, right!
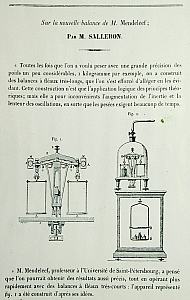
To do any of this you
used a lot of
precision apparatus. Glass tubes to hold the gases connected
to
other tubes of liquids so that pressures and volumes can be
measured. Scales (balances) of many types to permit you to
weigh
solids liquids and gases. Some of these were invented or
improved
by Mendeleev and their design was published, for some he followed the
designs of others.
I 'm not sure just what
this
apparatus is. My best guess is that it was used to separate
crude
oil into different fractions based on their volatility.
Some of the special
purpose glassware
on display.
Two balances.
The second was
used to compare the weights of equal volumes of gas. Because
the
glass containers are the same size they automatically compensate for
atmospheric buoyancy.
Two slow
pendulums. They may
have been used to measure equal time intervals so that reaction
kinetics could be studied.
The column of this
instrument had a
finely ruled scale. The device on it could be moved and its
height accurately determined. There was also what appeared to
be
a small telescope.
My best guess is that
this was used
to measure the change in the level of the liquid in the glass tubes
without requiring all of them to be scribed with scales. This
would ensure accuracy and save a lot of effort in the production of the
tubes.
This seemed to be a
transit minus the
telescope. I can't figure out just what it was used for in a
chemistry lab.
Here is Mendeleev's
office. We
saw reference books in Russian, French, German and other
languages. One in English was from the Franklin institute in
Philadelphia. Pictures of famous scientists were on the wall
behind him. A chess set was ready for a game and this glass
sphere tetrahedron was displayed on his desk.
Photography and painting
were
hobbies. Here is his camera and some of the pictures he
took. All photographers in those days were chemists but they
didn't have Mendeleev's expertise.
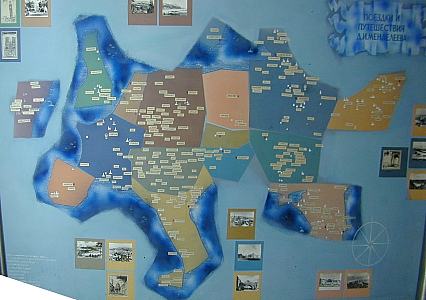
Mendeleev traveled
through much of
Europe and also the the United States. The map shows places
he
visited to teach, and to meet with other scientists to learn what they
had been working on.
His notebook entries
show some of the
places he visited in the US. The stop in Oil City was
especially
interesting to Nancy and I since it is only a few miles from where we
grew up.
A couple of bearded
scientists.
The one on the right is the famous one.
This little museum was a highlight of our trip to Russia.
If you want
more information
or can help me to understand more of what I saw I would like to hear
from you.
More
of our trip to Russia St.
Petersburg and Moscow
See other places we have
visited here.
<>Go
to our Personal
home page
Go to our Community
page
Go
to our Science
Fun page
E-mail Nancy
and
Alan