Pikeville Science and Math
Camp 2013 week 1
Chemistry
Monday campers
learned safety rules that would apply in all their classes through the
week.
They decorated a pair of safety glasses with their own choice of colors.
The glasses were for their use throughout the week and then they could
take them home.






Some of our volunteers were making tails for the moles the
campers were to put together.
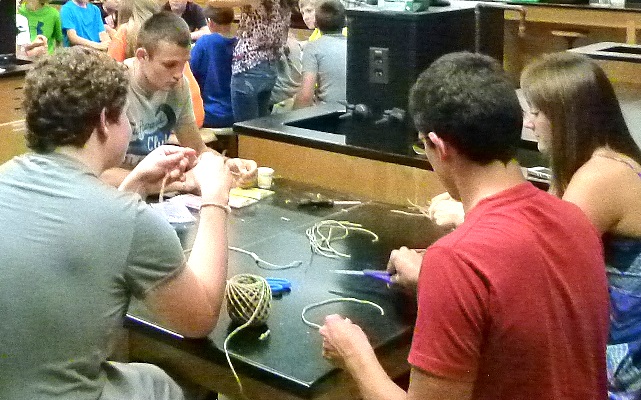
Building moles.
These little stuffed ones were to remind the kids of the chemical mole.
If you need a reminder yourself it is the number of grams of a chemical
that is equal to that chemical's atomic weight.
That's 6x10
23 molecules.
They also checked what a mole of various other substances looked like.


And some after they are done.

Slime made from polyvinyl alcohol and borax solutions.
You can imagine polyvinyl alcohol (PVA) molecules as resembling limp
strands of spaghetti that are as long as a football field.
When dissolved in water they easily slide over each other.
The sodium borate molecules are much smaller, imagine pinhead size.
Each solution is nearly as runny as water.
However, when they are mixed the borate ion forms links with random
spots on the PVA molecules and the mix becomes very gooey.
The mix is still almost all water (96%) so it is surprising that it's
consistency has changed so much.




Astronomy
Physics
Here we are
lighting a fluorescent bulb using static electricity.
Take an aluminum pie pan, tape it to a plastic straw, and hold it near
a balloon that has been given a static charge by rubbing it on some fur
(or beard).
Touch the pan while it is close to the balloon.
That gives a path to ground for the electrons that are being repelled
from the balloon.
Take your hand away from the pan and no electrons can move to or from
it because the straw is a good insulator.
Then touch the bulb to the pan and the bulb will blink as electrons
from the person holding the bulb return to the pan.
The third picture shows a blink.



Ben Franklin would have recognized the electroscope I am holding even
though we have used different materials for all of the parts.
Construction is simple, a container with a conductor through the top
that has two pieces of foil leaves attached to it's bottom end.
When the electroscope is charged the two foils repel each other and
separate.
Ben used a glass jar I used plastic.
He used linen strings and later gold foil for the sensor, I used
aluminum (a metal that wasn't known in his time).
He used a metal rod to hold the foils and I used a paper clip.
The kids made their own electroscopes with a plastic bottle, a piece of
aluminized mylar folded to make two leaves, and a paper clip.
As a charged balloon or pie pan was brought close the mylar leaves
would seperate.




We then turned plastic strips and campers into electroscopes using a
Van de Graaff.
It is misspelled on the board behind them.
When they were charged their hair was repelled and stood out in all
directions.

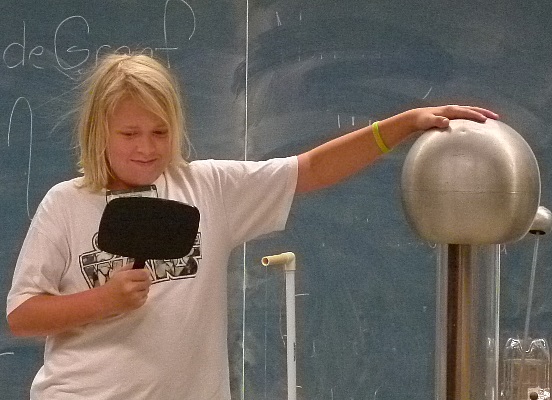
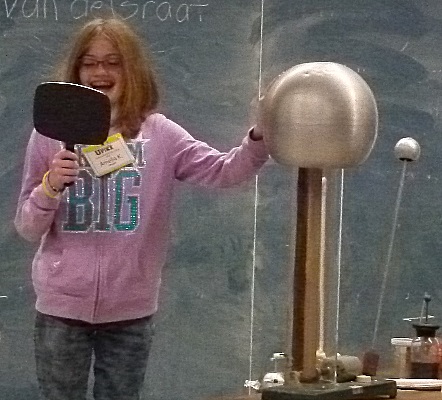

They wanted to see what would happen to me too.

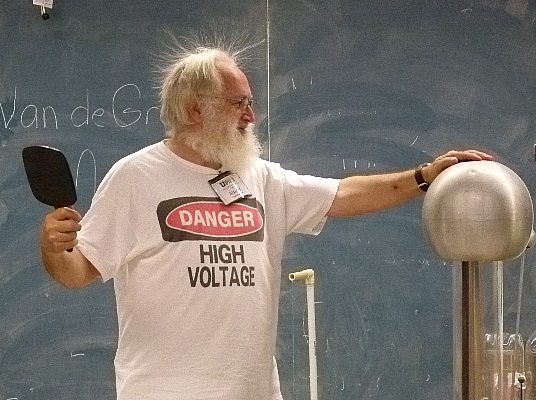
Then they got to see what electricity feels like.
They made a chain with fluorescent bulbs between the kids so thy could
see them light up as the current passed through them.
The lights were dimmed for some of the pictures so the light from the
bulbs would show up better.
We use a pan to make contact with the Van de Graff to make it easy for
the first person in the line to control the size of the spark and the
charge that they all feel.
You might say that they are "in charge".


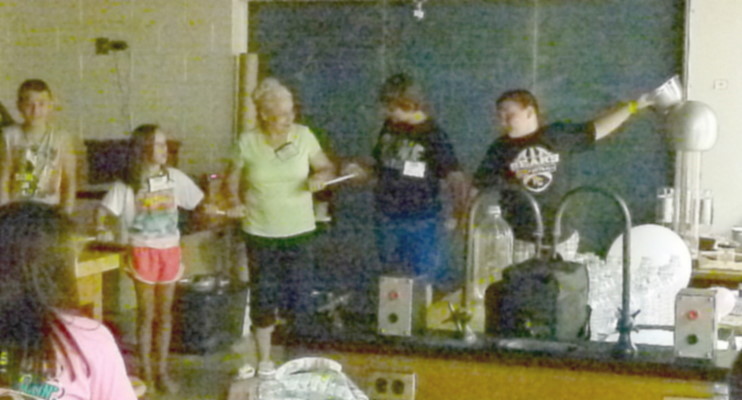
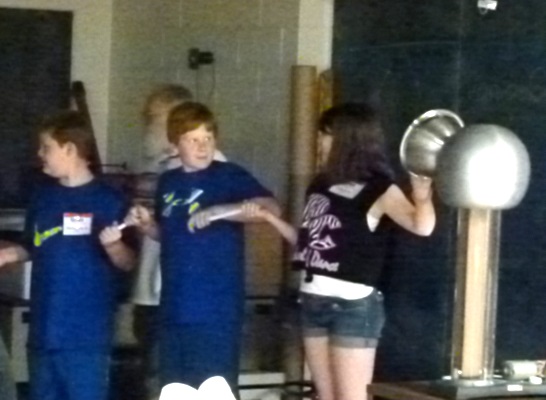
A string of metalized beads on an insulating string is another way to
get a charge from the Van de Graaff.
As they slide their hand along the beads the insulation provided by the
string will eventually be insufficient to hold back the charge and a
spark will jump between each of the beads and to their hand.
The anticipation can be as bad as the shock.

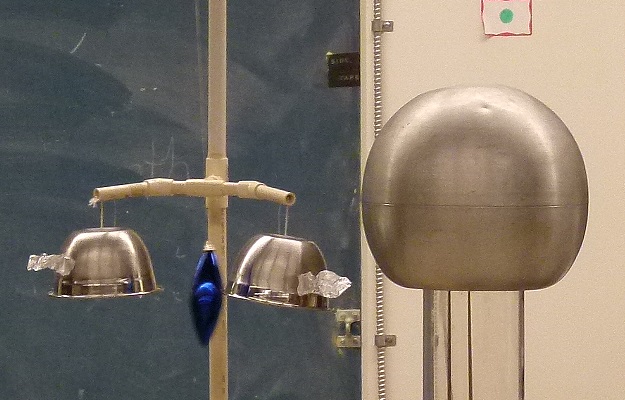
The apparatus on the left is called Franklin's bells.
A charge on the bell closest to the Van de Graaff is charged because of
a coronal discharge from the piece of foil attached to it.
The blue bob is attracted to it but as soon as it touches it has the
same charge and is repelled.
Then it swings over and touches the other bell, is discharged and the
process repeats.
We devoted one class to the study of sound.
We used a long spring as a model of how sound travels through the air
as a compression wave and how it is represented on an oscilloscope
(sorry no pictures).
The spring also is a good model of an echo as the wave bounces off its
end.
Compression waves work in solids too.
This aluminum rod can have sound waves bouncing from one end to the
other.


Anything that vibrates can produce sound waves though some may be too
high or low for us to hear.
By making controlled vibrations in the range of human hearing we can
make music.
Popsicle sticks are the source of vibrations in this kalimba all the
kids made.
By adjusting the length of the sticks they can be tuned and they could
play simple tunes.


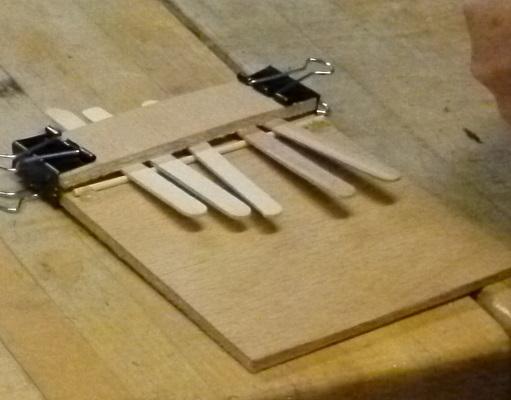
Three of the sound sources the kids tried.
A completed and tuned kalimba.
The cup has a strip of plastic that will say "Science is fun" when you
slide your fingernail along it.
The wood has a piece of fish line that can be played as a guitar or
violin.
The vial from chemistry class holds 18 grams of water, one mole.
We also investigated resonance (periodic motion enhanced by addition of
small amounts of energy in phase with the motion) at laboratory scales.
Then we took a look at one of the biggest engineering failures due to
the resonance, the Tacoma Narrows Bridge.
We had the kids bring in their music players and earphones or ear buds.
They set them to their normal listening level and we measured the sound
level.
Then we had them turn them up to maximum and measured the output again.
Most of this group had their players set at safe levels for normal
listening but 6 of the 42 had theirs set at 90 dBa or higher .
In a work environment an employer would have to provide protection for
that level of sound.
When set at maximum 3/4 of them put out unsafe levels with the highest
being 123 dBa or higher.
That is above the threshold of pain and where very brief exposures can
cause hearing damage.


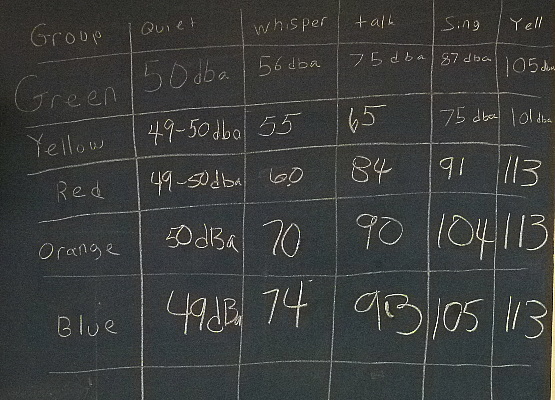
We had the kids in each of the groups be as quiet as possible, whisper,
talk normally, sing, and then cover their ears and yell.
Here are the results.
When you consider that the red and yellow groups had fewer kids by
nearly a half they put in a good showing.
On Wednesday the kids made two kinds of accelerometers.
One measured horizontal and the other vertical acceleration.
They could use one to determine the forces in a car going around a
curve and the other when they were on a trampoline.
They could also try them when we go on rides at the amusement park on
Friday.



Biology
Computers
The computer class
started with learning binary numbers and how computers use them.
Binary numbers are represented using only the digits "0" and "1".
By assigning different values for each column higher numbers can be
represented.
Starting from the left the column values are 1, 2, 4, 8, 16, 32, ...
The number number the kids are holding in the last picture "01010" is
no 16s, an 8, no 4s, a 2, and no 1s which make a total of 10 in our
usual way of writing numbers.

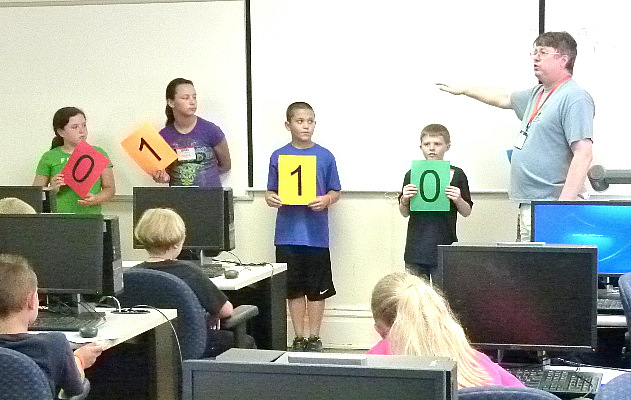
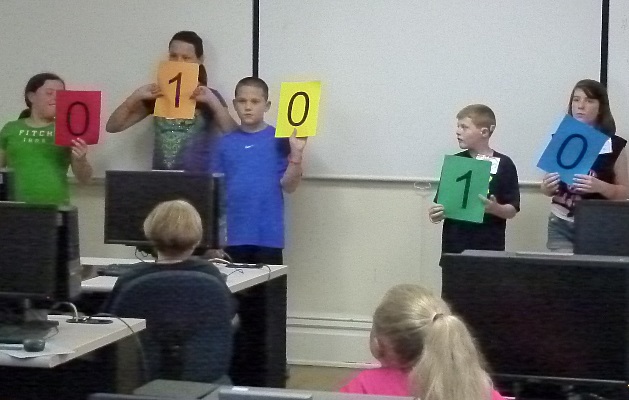
Binary numbers are used to represent all sorts of data, from numbers,
to music, photos, videos, and anything can be measured.
To limit errors a method of adding additional data to let you discover
when a bit of data has been changed.
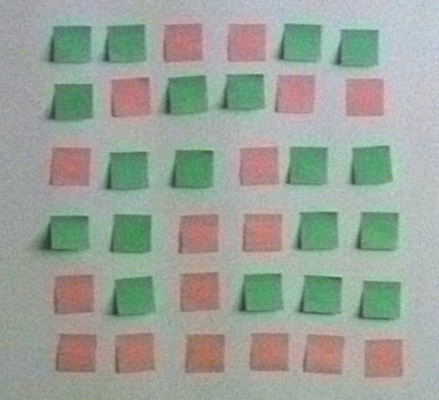
These Post-its represent 5 binary numbers and the added information
needed to discover which one has changed.
Originally each of the rows and columns had an even number of each of
the colors.
When one of the Post-its in the array is changed the number of each
color in that row and column is now odd which allows you to recognize
the one that has changed and then change it back.
Actual error correction schemes are more complicated but this
illustrates how they work.
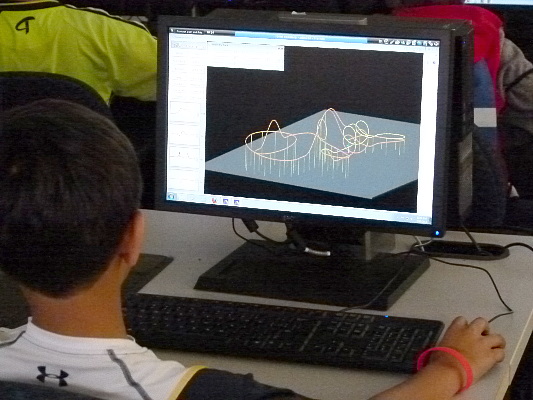
The kids used computer programs to design roller coasters.
The program then evaluated them to see if they would work, what the
maximum G loads (accelerations) would be and would the passengers
survive.




This picture from a past camp shows what two of this year's instructors
looked like in 2001.
One of them is the daughter of the computer instructor.
Lunch
I can't overlook
this important part of the day.
The new cafeteria is open, bright and much bigger than the one we ate
in previously.


Construction on the campus meant we had to detour through another
building to get there.
Contest
During the week
the campers were tantalized by the prizes that were to be awarded.
These are the first through fifth prizes for the straw tower
competition.
And a few of the entries.
Some marvelous designs.

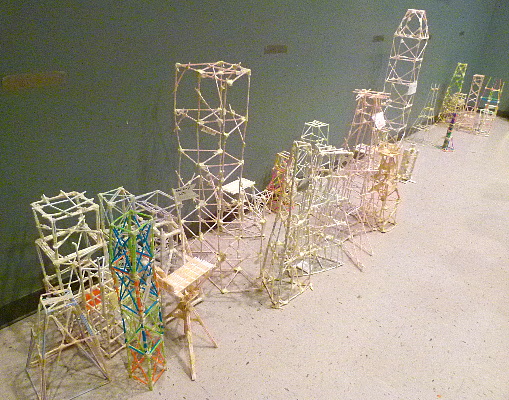
Testing how many marbles it could hold was nerve wracking for the kids.
The greatest height multiplied by the number of marbles it held was the
score.



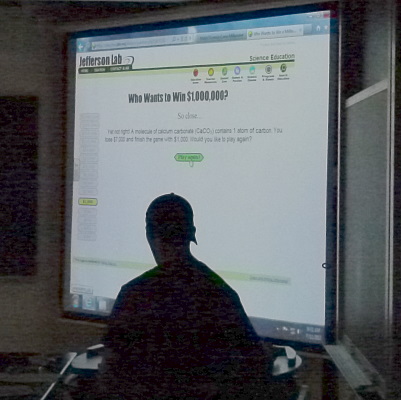
The Who Wants to Win $1,000,000 game presented the campers with science
questions.
They would get harder with each round.
The foam rockets they built in Astronomy class were used in a game of
rocket golf.

Design and build a paper bridge then test it to destruction to win
points.
The more it held the higher your team's score.
There were lots of other contests too.


In the left turn airplane contest your score depended on how far down
the hall it went after you threw it out a classroom door.

Use a rubber tubing catapult to fire four water balloons at a
target.
It takes three people to launch the balloon.
Only the last balloon counts for your score so use the first three to
get range and bearing.

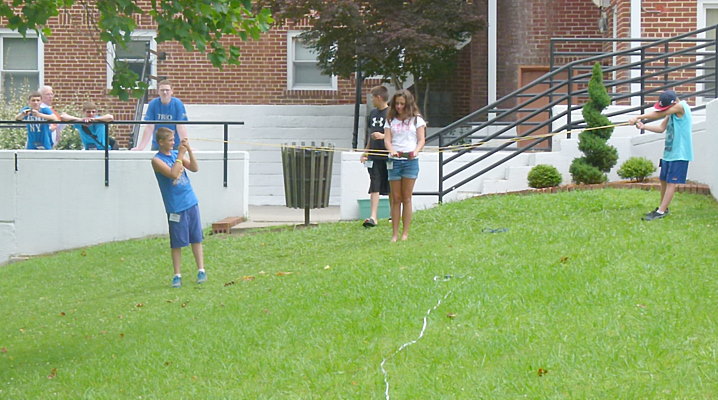

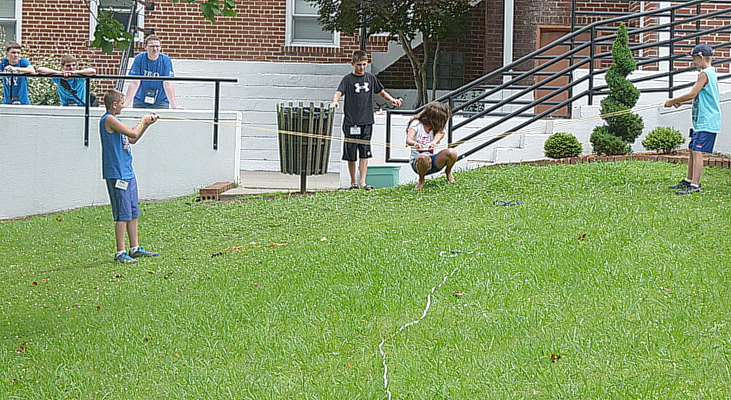
Games
We had a few games
and puzzles for the kids to play if they finished their contest round
early.
They tried to solve the eight queens, tangrams, and match stick puzzles.
This one is the game of Nim.
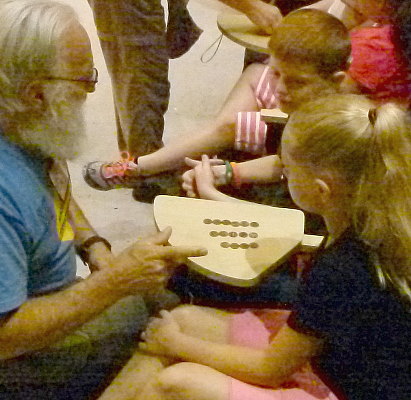
The winners
Field trip to Camden Park
Campers
and helpers are ready to load up for the trip.

 On to the buses
On to the buses


 At the park.
We got to study the physics of the
rides and we all had fun.
At the park.
We got to study the physics of the
rides and we all had fun.





















 We all had a great time this
week.
I hope that you enjoyed seeing a
little of what we did at camp.
Check out some of the links below if
you have time.
We all had a great time this
week.
I hope that you enjoyed seeing a
little of what we did at camp.
Check out some of the links below if
you have time.
Go to our Science Fun
page
Go to our Travels page
Go to our Personal home
page
Go to our Community page
E-mail Nancy and Alan

The
www.mrtc.com/anvk/ web site by Alan
Kuehner is licensed under a Creative
Commons License.
Additional information and permissions beyond the scope of this license are available here.


